Radiochemie München RCM in co-operation with Isotope Technologies Garching GmbH (ITG)
Scientists: Dr. Christoph Barkhausen, Dr. Konstantin Zhernosekov
Funded by Bayerischen Forschungsstiftung (BFS)
Through successful clinical applications in the field of Endoradiotherapy and Brachytherapy there is growing demand for the reactor nuclide 177Lu worldwide. However, very high claims regarding the quality of the nuclide complicate the production process and thus the accessibility.
Aim of this project was the development of a chemical method to produce non carrier added (n.c.a.)177Lu at the high neutron flux source FRM II in Garching and to establish the first European production according to the German Medicine Law (Arzneimittelgesetz). For this purpose preparative chromatographic methods were developed und applied. Subsequently, the process was transferred to a hot cell to establish a routine production of 177Lu at an industrial scale.
The low-energy β--emitter 177Lu (T1/2 = 6.71 d) represents an ideal vehicle for selectively deposing high energies within small volumina. Its physical characteristics are implemented in radioimmuno therapy and peptide receptor radionuclide therapy (PRRT) with promising results mainly applied to cure malignant diseases. A successful application of the reactor nuclide 177Lu is limited by the production-related specific activity of the nuclide [Bq/mg] and its purity. A high specific activity of the radionuclide is mandatory to achieve a high specific activity of the radiopharmaca and thus an optimally applied amount of the latter. If no high specific activity and purity is achieved, a negative influence regarding the production of the therapeutic agent will occur.
177Lu can be produced through the following nuclear reactions:
(1) 176Lu(n,γ)177Lu;
(2) 176Yb(n,γ)177Yb →177Lu
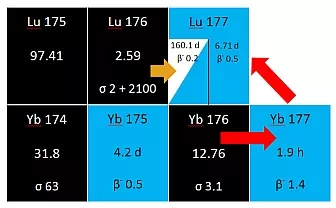
Figure 1:Cut-out from the chart of the nuclides; reaction paths to produce <sup>177</sup>Lu: yellow arrow indicates the direct production path, the red arrows show the indirect reaction path
The first nuclear reaction (1) represents a neutron capture reaction of 176Lu, which leads to carrier added (c.a.) 177Lu and consequently to a lower product quality. In addition, seen from a medical point of view and under radiation protection aspects, through irradiation of 176Lu the unwanted long-lived radionuclide 177mLu is produced (T½ = 160.1 d) (Fig. 1). The fraction of obtained 177mLu can reach up to 0.1 % of the overall 177Lu activity. With regard to its use on human beings and given the high amounts of activity that have to be produced, this contaminant has to been seen very critically. Due to the long half-life of the nuclide a continuing risk of its release into the environment exists. Furthermore, the user in the hospital faces the problem of safe-handling and radioactive waste management of the remaining quantities of 177mLu, which will hardly be solved by storage like it is common in hospitals nowadays.
The more reasonable alternative seen from a medical and commercial approach is the production of n.c.a .177Lu via the indirect nuclear reaction (2). By irradiating highly enriched 176Yb (> 99%) the short-lived radioisotope 177Yb (T½ = 1.9 h) is produced and decays to 177Lu (Fig. 1). In this case, the target nuclide 177Lu is an isotone of the irradiation target 176Yb and can therefore be chemically isolated in its non carrier added form. As no 177mLu is generated through the decay of 177Yb, 177Lu is obtained with a very high nuclidic purity. A drawback of this strategy is the mandatory radiochemical procedure to separate macro quantities of Yb from micro quantities of 177Lu. As the desired radionuclide and the target nuclide represent two adjacent elements of the lanthanide series the separation poses a great challenge because of their high chemical similarity. Moreover, the use of massive 176Yb targets complicates the task even more.
Based on cation exchange chromatography a process was developed with which target masses up to 150 mg can be handled obtaining yields of n.c.a.177Lu up to 95 %. The separation system evaluated at RCM has been successfully built up at ITG within a Hot Cell as a semi-automated process. The routine production of n.c.a.177Lu at an industrial scale was established, including the handling of a highly active target, the chemical isolation of 177Lu and the transfer of the product into the desired chemical form. In order to obtain pharmaceutical quality of the product and the manufacturing license the whole system was transferred to a cleanroom environment.